IRIS is a single-use, skin-mounted needle guide that improves targeting, reduces confirmation scans, and shortens procedure time—without capital equipment or workflow disruption.
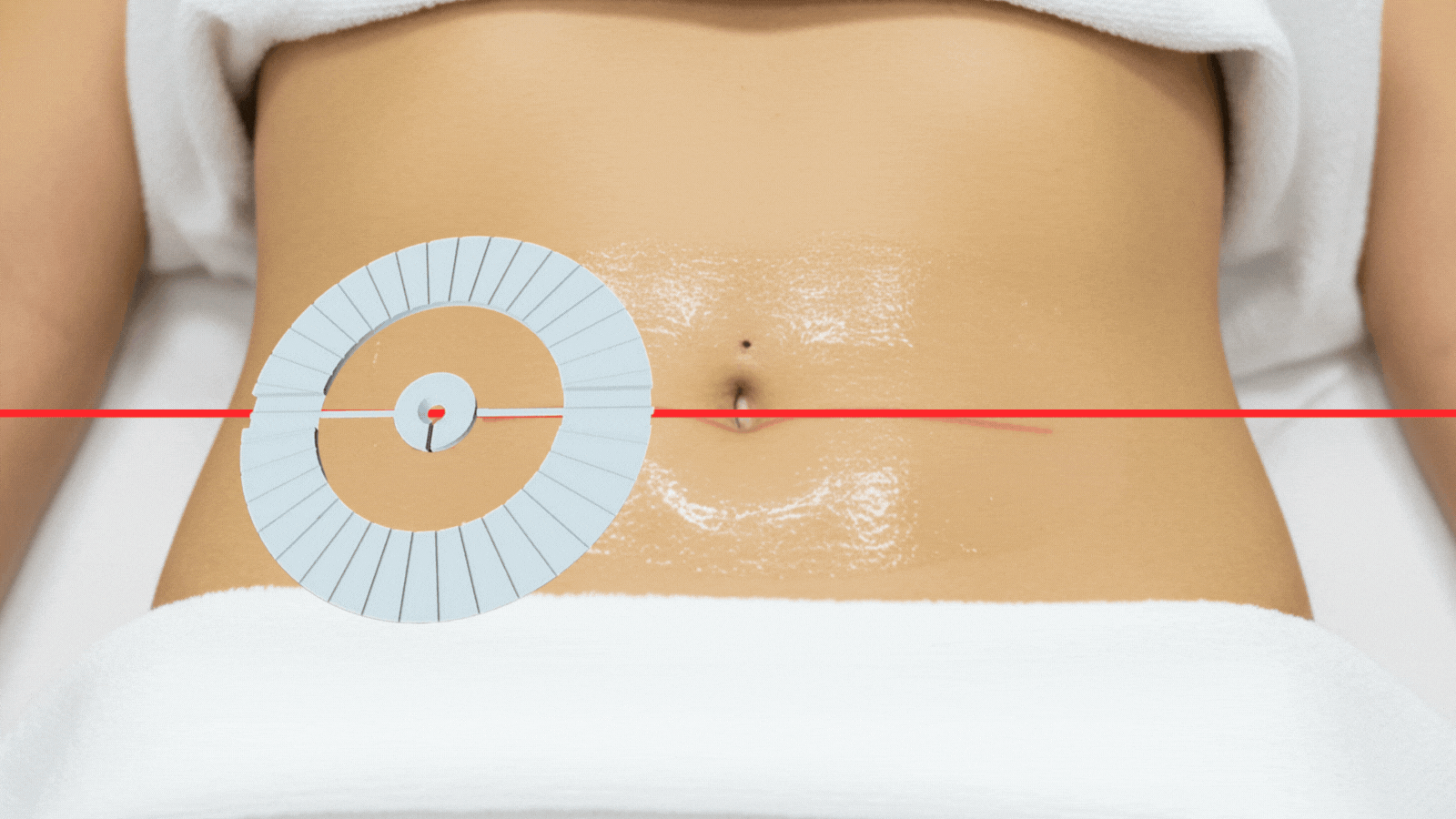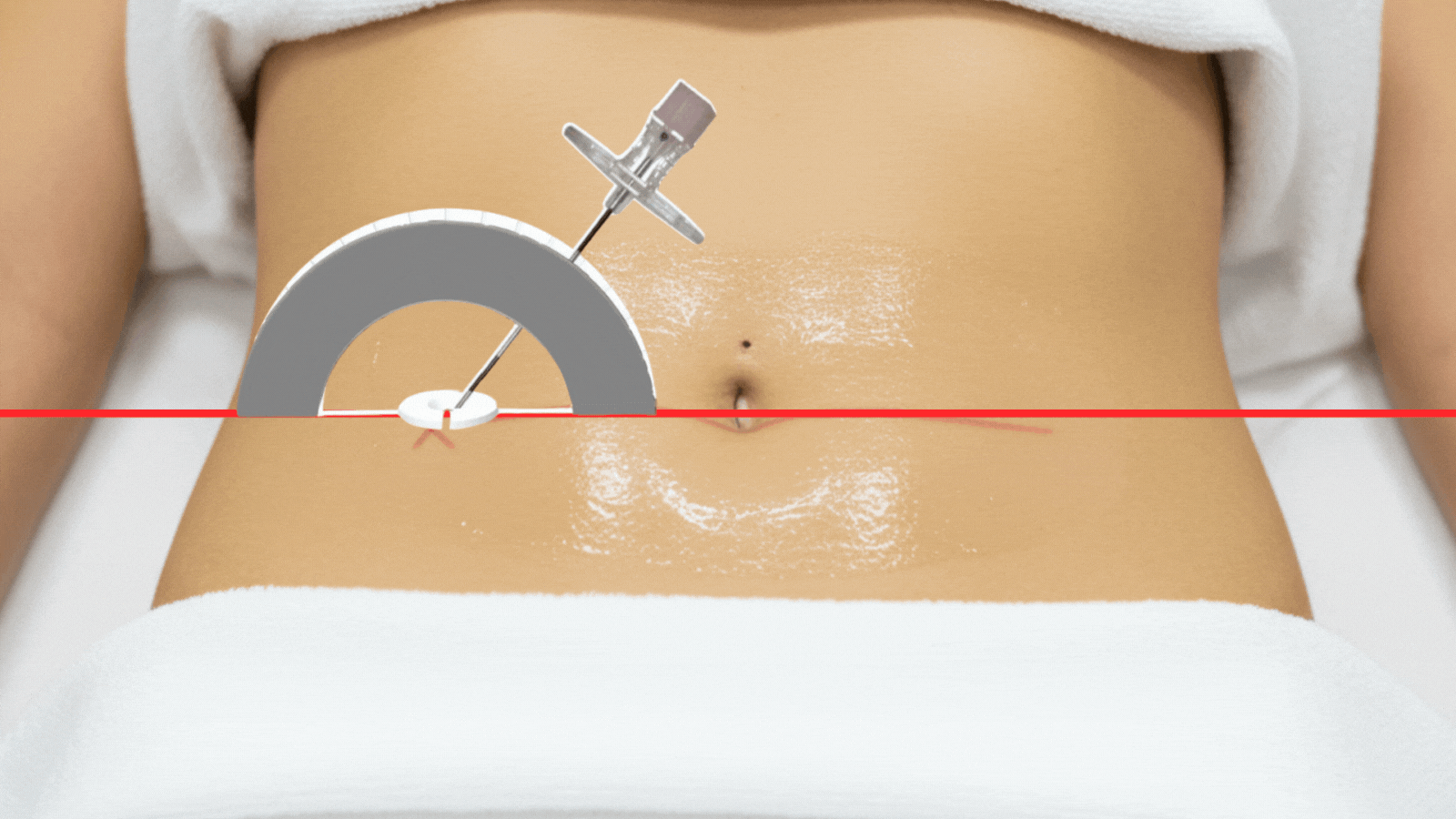
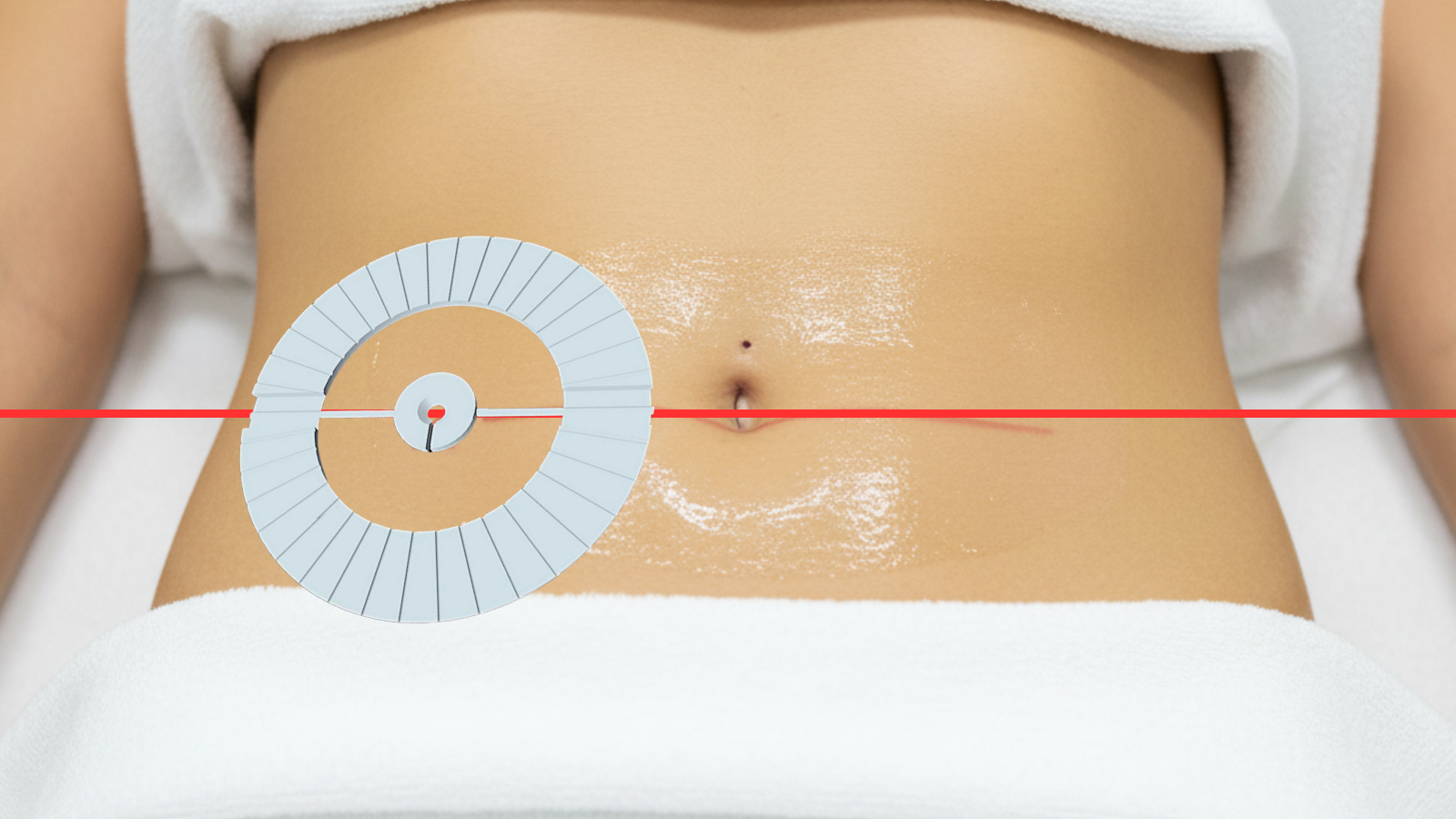
From procedure planning to needle placement, IRIS delivers confidence and efficiency at every step
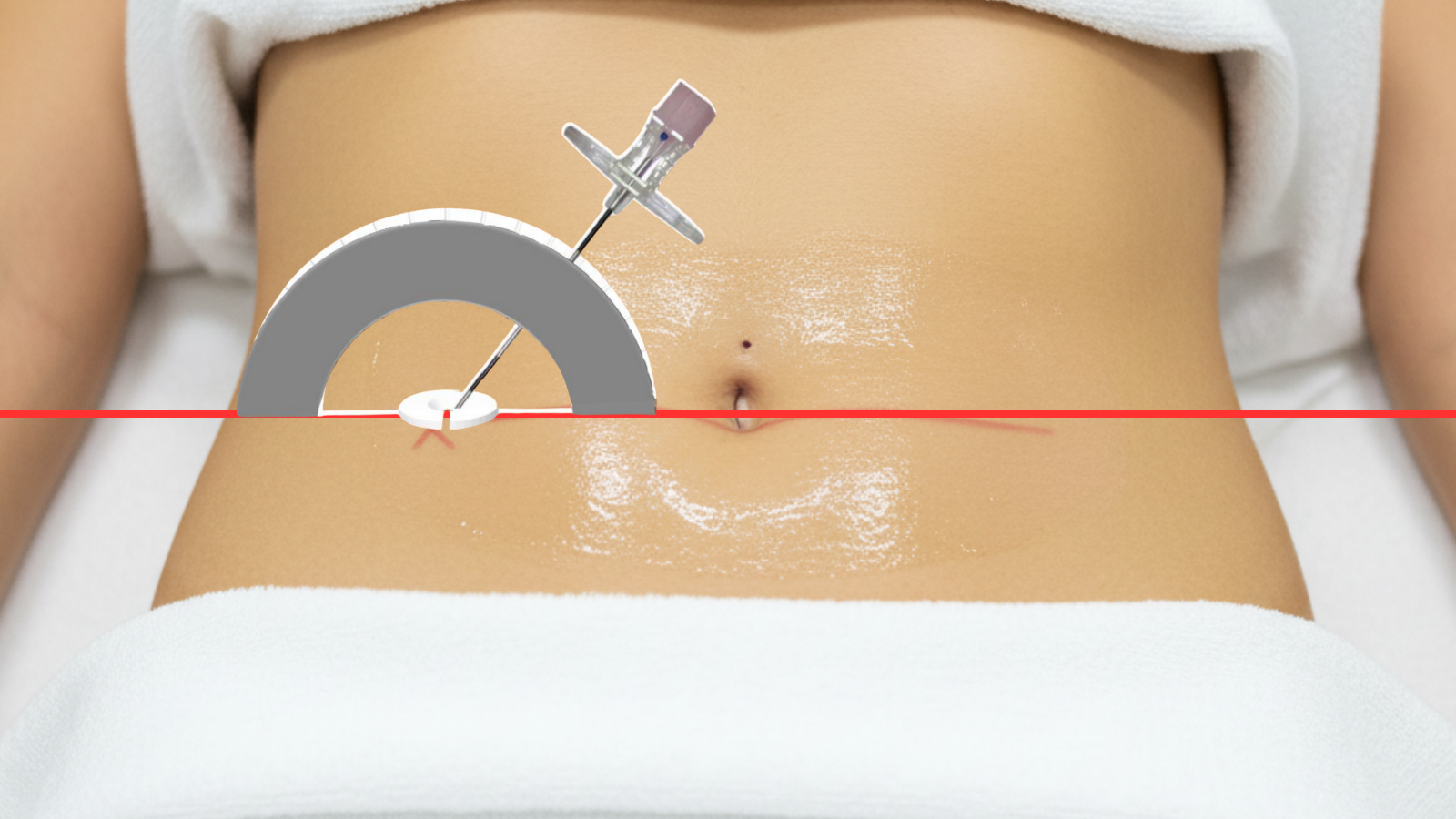
Align IRIS on skin entry site.
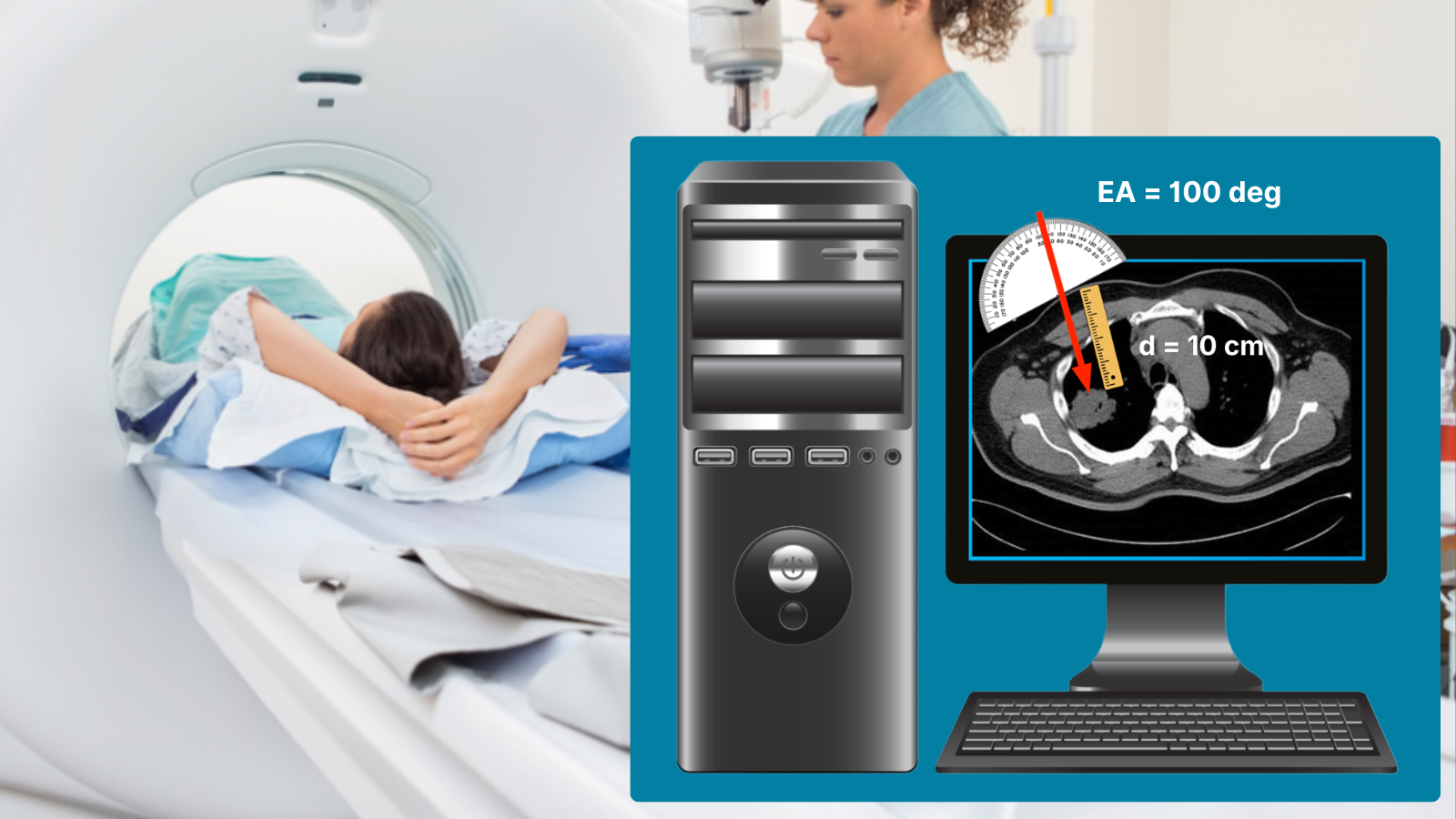
Identify distance and entry angle to lesion.
Position needle to calculated angle & depth.
Incubated at Vanderbilt University Medical Center

Founder & CEO
Assistant Professor of Neuroradiology at Vanderbilt University and an interventional radiologist with over a decade of CT-guided procedure experience. Dann trained at Johns Hopkins, Tufts, Lahey, and Stanford and is passionate about simplifying biopsy workflows to improve patient outcomes.

Co-Founder & COO
Co-Founder and COO of IRIS and Entrepreneur-in-Residence at Vanderbilt University Medical Center. Jon was previously CMO at Spacious (acquired by WeWork) and Marketing Manager at Google Cloud, with a background spanning healthcare, venture capital, and SaaS commercialization.
We are looking for Interventional Radiologists to help test and pilot IRIS
Regulatory Disclaimer: IRIS is an investigational device currently in the research and development phase. It has not been cleared or approved by the U.S. Food and Drug Administration (FDA) and is not available for clinical use, patient care, or commercial sale. Any information provided on this website is for educational and research purposes only and should not be interpreted as medical advice.